Abstract
Background:
Persons living with HIV (PLWH) have an increased risk of B-cell malignancies, including diffuse large B-cell lymphoma (DLBCL), even with well-controlled HIV. The lifetime cancer risk in PLWH is 25% to 40%1-3, and cancer is the leading cause of death in PLWH in economically advanced countries. Non-Hodgkin lymphoma (NHL) is the most common cancer in PLWH in the United States. Despite aggressive frontline treatments, about 20-30% of patients with HIV-associated B-NHL will not be cured with current therapies4, comparable to the non-PLWH B-NHL population.
Axicabtagene ciloleucel (axi-cel) is an FDA-approved CD19-directed CAR-T cell product for the treatment of patients with relapsed or refractory (R/R) DLBCL based on the ZUMA-1 (>2 prior lines of therapy5) and ZUMA-7 (primary refractory or relapse within 12 months of initial therapy6) trials. As patients with HIV were excluded from registrational studies, only sparse case reports or small case series7 exist to guide treatment in this context. With regards to safety concerns, a study of CD4 cell count trends in a subset of HIV-negative patients receiving axi-cel demonstrated low CD4 cell counts (median baseline 220/μL) that decreased over time and remained persistently low after lymphodepletion and axi-cel therapy (median CD4 cell count 155/μL at 1 year post treatment).8
This critical gap in evidence can potentially lead to lives lost in patients with HIV, typically aged 25 to 65 years. We hypothesize that axi-cel will be safe and feasible in treating R/R HIV-associated DLBCL.
Study Design and Methods:
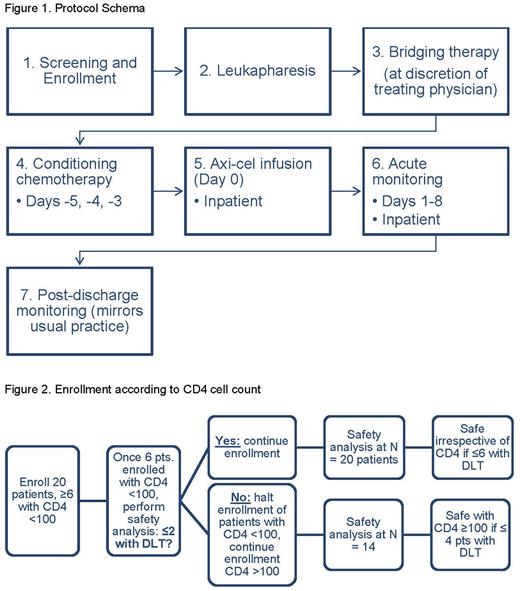
This is a multicenter phase I study to define the safety and feasibility of axi-cel in R/R HIV-associated DLBCL (NCT05077527). Concurrent correlative studies will investigate immune reconstitution and HIV virologic response. All participants will receive the standard dose of axi-cel at AMC sites following conditioning chemotherapy; bridging therapy may be given at the discretion of the treating physician (Figure 1).
Key eligibility criteria include HIV positivity with viral load <50 copies/mL; diagnosis of R/R DLBCL, high-grade B-cell lymphoma, primary mediastinal B-cell lymphoma, or follicular lymphoma grade 3B; either post 2 prior lines of therapy or refractory/relapsed within 12 months of frontline treatment; evaluable disease; adequate organ/marrow function; and CD19 positivity confirmed if previously received CD19-directed therapy.
The study will enroll 20 participants, including at least 6 participants with a CD4 cell count <100/μL. A safety assessment of participants with CD4 cell count <100/μL will be done after 6 participants in that cohort have been enrolled in this study (Figure 2). If >2/6 participants experience dose-limiting toxicity, the study will halt recruitment of participants with CD4 cell count <100/μL and continue to recruit participants with CD4 cell count of ≥100/μL.
The primary endpoint of the study is safety and feasibility. Safety is defined by blood count recovery of neutrophils to grade ≤2, and platelets to grade ≤2 within 6 weeks of axi-cel infusion, incidence/severity of infection, and incidence/severity CRS/ICANS9 among patients who receive axi-cel. The incidence of toxicity will be summarized using frequency and percentage and presented overall and for each CD4 cell count cohort (<100 versus ≥100/μL). The feasibility assessment will be evaluated as the proportion of participants who receive axi-cel among all enrolled participants who undergo leukapheresis. Based on published data10 from patients without HIV (success rate ~85%) and anticipating potential difficulties in clinical progression, leukapheresis, and manufacture, we assigned >50% as the acceptable feasibility cutoff for our study population.
Key secondary endpoints include 1) complete response (CR) rate per Lugano criteria,11 2) event-free survival (time from enrollment to earliest of disease progression; commencement of new anti-lymphoma therapy; or death), and 3) response duration (time from date when response criteria are met to relapse or progression). Key exploratory endpoints include 1) differences in cytokine profiles between patients who achieve CR and those achieving <CR per Lugano criteria (tested for statistical significance using Wilcoxon rank sum test) and 2) associations between cytokine profile and CRS and ICANS severity (≥ grade 3 vs < grade 3, tested for statistical significance using Wilcoxon rank sum test).
Disclosures
Barta:Seagen: Honoraria; Acrotech: Honoraria; Daiichi Sankyo: Consultancy; Affimed: Consultancy; Kyowa Kirin: Consultancy, Honoraria; Janssen: Other: Independent Data Monitoring Committee member. Palomba:Beigene: Consultancy; Ceramedix: Consultancy; Juno: Patents & Royalties; Kite: Consultancy; MSKCC: Patents & Royalties; MustangBio: Consultancy; Nektar Therapeutics: Honoraria; Notch Therapeutics: Consultancy; Novartis: Consultancy; Pluto Immunotherapeutics: Honoraria; Rheos: Honoraria; Synthekine: Consultancy; Vor Biopharma: Honoraria. Noy:Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal